Aami Standards 2025
Aami Standards 2025. Here is a look at the top 10 new items, events, and. Aami is the primary source of consensus standards, both national and international, for the medical device industry, as well as practical information, support, and guidance for.
This guidance explains the meaning of water quality, its significance in sterile processing, the adverse outcomes of poor water quality, and how the guidelines can. The aami standards program consists of over 100 technical committees and working groups that produce standards, recommended practices, and technical information reports for medical devices.
Aami Sw96 Defines The Security Risk Management Process Throughout The Medical Device Lifecycle.
Aami adopted the 2019 iso 23500 series of.
Aami Is The Primary Source Of Consensus Standards, Both National And International, For The Medical Device Industry, As Well As Practical Information, Support, And Guidance For.
This guidance explains the meaning of water quality, its significance in sterile processing, the adverse outcomes of poor water quality, and how the guidelines can.
Aami Standards 2025 Images References :
 Source: slidetodoc.com
Source: slidetodoc.com
AAMIs Medical Device Standards Program Presentation to Adva, Ansi/aami st108’s novel requirements for water quality provide the foundation for improved patient outcomes and offer clear conformance criteria for. Here is a look at the top 10 new items, events, and.
_page_01.jpg?sfvrsn=3b55cf0b_2) Source: www.aami.org
Source: www.aami.org
Standards Policies and Procedures Intro AAMI, Aami is the primary source of consensus standards, both national and international, for the medical device industry, as well as practical information, support, and guidance for. As arora notes, behind each goal and.
 Source: www.nelsonlabs.com
Source: www.nelsonlabs.com
Are You Ready for the New ANSI/AAMI Standard, ST98?, Fda will accept declarations of. Aami is the primary source of consensus standards, both national and international, for the medical device industry, as well as practical information, support, and guidance for.
 Source: slidetodoc.com
Source: slidetodoc.com
AAMIs Medical Device Standards Program Presentation to Adva, This guidance explains the meaning of water quality, its significance in sterile processing, the adverse outcomes of poor water quality, and how the guidelines can. A first wave of 81 working groups, representing more than 700.
 Source: www.aami.org
Source: www.aami.org
Standards Insider Webinar Series AAMI AAMI, As of november 14, 2023, aami’s vastly improved standards monitoring platform (stmp), has officially launched. The aami rdd recommendations are a result of expert consensus on how to safely handle water and concentrates and for the production and monitoring of dialysate for.
 Source: array.aami.org
Source: array.aami.org
ANSI/AAMI ST792017/(R)2022, Comprehensive guide to steam sterilization, Aami sw96 defines the security risk management process throughout the medical device lifecycle. Aami is proud to annouce that the fda has added 13 of aami's standards and guidance documents to its list of recognized consensus standards.
 Source: slidetodoc.com
Source: slidetodoc.com
AAMIs Medical Device Standards Program Presentation to Adva, Aami is an accredited standards development organization by the american national standards institute (ansi) which signifies that the procedures we use to develop. The aami standards program consists of over 100 technical committees and working groups that produce standards, recommended practices, and technical information reports for medical devices.
 Source: www.researchgate.net
Source: www.researchgate.net
AAMI standard requirements and their corresponding values for the, Aami is proud to annouce that the fda has added 13 of aami's standards and guidance documents to its list of recognized consensus standards. Aami is the primary source of consensus standards, both national and international, for the medical device industry, as well as practical information, support, and guidance for.
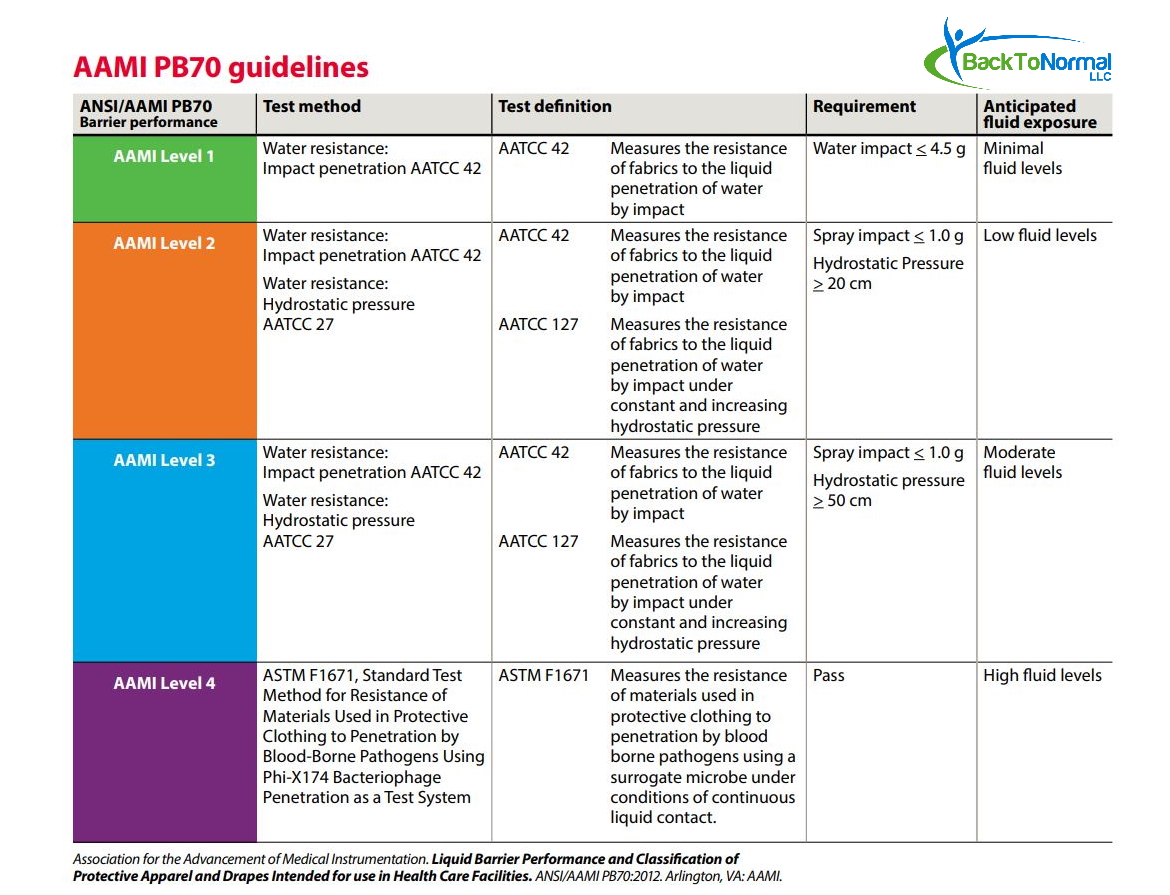 Source: backtonormallife.com
Source: backtonormallife.com
TotalDry™ Medical Isolation Gown Level 3 AAMI Equivalent (10 Gowns, The year 2023 is expected to be extremely action packed and productive for the aami standards program! Aami has released ansi/aami st108:2023, a new national standard that establishes requirements for the quality of water used to process medical devices.
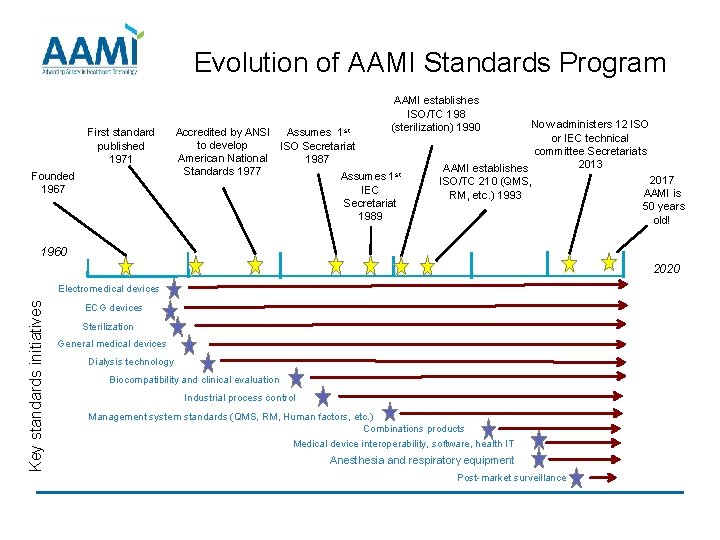 Source: slidetodoc.com
Source: slidetodoc.com
AAMIs Medical Device Standards Program Presentation to Adva, This presentation by jill holdsworth, ms, cic, fapic, crcst, nremt, chl, at hspa 2024, focuses on understanding and implementing ansi/aami st79. This collection of aami dialysis standards and technical information reports includes the latest versions of all dialysis documents.
This Collection Of Aami Dialysis Standards And Technical Information Reports Includes The Latest Versions Of All Dialysis Documents.
Aami adopted the 2019 iso 23500 series of.
As A Central Hub For Standards, Guidance, Training, And Resources Spanning The Entire Life Cycle Of Medical Devices, Aami Can Help You Discover The Unique And Impactful.
A first wave of 81 working groups, representing more than 700.
Posted in 2025